EMINVEST Pharmaceuticals
Committed to excellence
Founded in 1992,
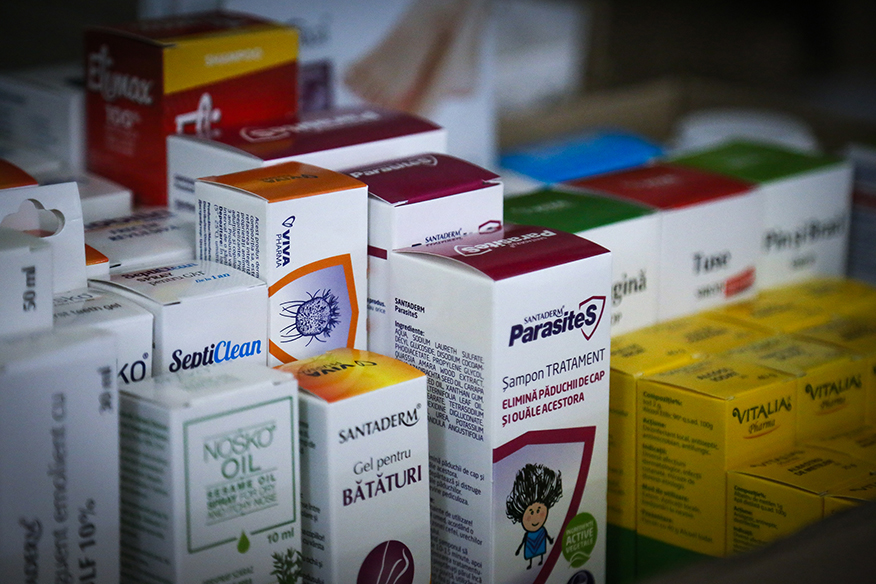
EMINVEST Pharmaceuticals is an innovative Romanian certified company specialized in research, development, production and commercialization of a diverse range of OTC products, registered as Medical Devices, Biocides, Cosmetics and Supplements, across 11 EU countries.
Our adaptability positions our company as a reliable partner capable of addressing unique market demands and ensuring client satisfaction through custom solutions.
As a trusted private label manufacturer, EMINVEST Pharmaceuticals upholds its reputation for excellence, offering a blend of experience, innovation and flexibility to meet clients’ needs.
Safeguarding the well-being of the consumers is one of our main missions.

SRAC ISO 9001:
Quality Management System

SRAC ISO 13485:
Medical Devices Quality Management System

SRAC ISO 22000:
Food Safety Management System

SRAC ISO 22716:
Cosmetics GMP
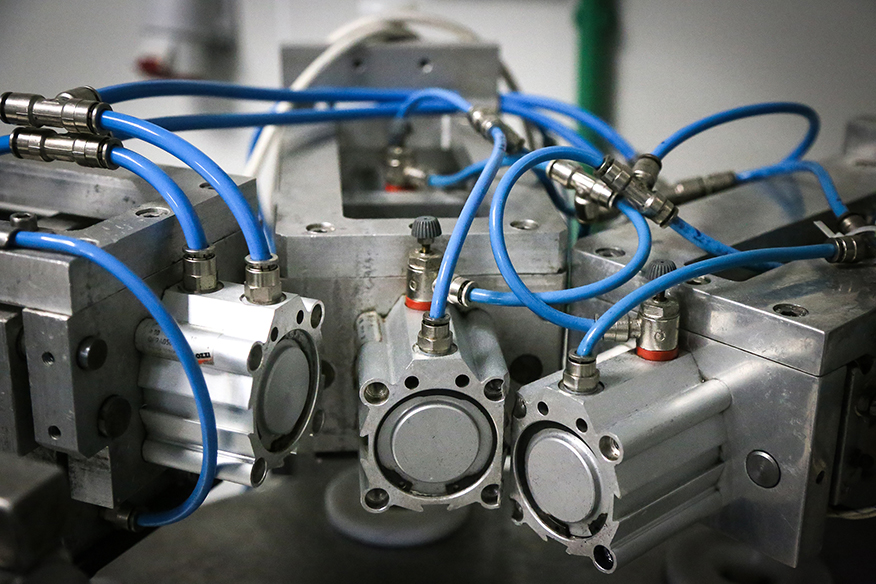
Our production lines
EMINVEST Pharmaceuticals is committed to maintaining a controlled and clean environment for the production process. Our performant production lines ensure the highest standards of quality and compliance.
Over the years, we have developed more than 400 dossiers. EMINVEST is actively engaged in an audit process to update production lines and medical device dossiers, aligning them with the requirements of the new Medical Device Regulation (MDR).
Our team’s proficiency in providing tailored solutions attests our experience in raw material procurement, processing and product development. The personalized products we can formulate are available in diverse doses and packaging formats.
Why EMINVEST Pharmaceuticals?
We operate based on core values that prioritize transparency every step of the way. Our commitment to delivering high-quality products and services is matched by our dedication to providing precise tailored solutions.
By choosing us, you will benefit from our team's expertise fueled by our relentless pursuit of precision, efficiency and customer satisfaction.
Our team of professionals delivers top-notch products and services due to our constant progress
We tailor our solutions in accordance with every client’s needs
Providing a complete range of services: research & development, production, certification, logistics
Adaptability is one of our key features – we find and implement proper solutions in each case
We handle requests and processes in a timely manner
Our know-how and modern production lines guarantee high performance at the correct cost
The entire developing and production process is transparent